Artificial Intelligence Can See Breast Cancer Before it Happens
Key Points
- Our image base deep learning model identified unique signals of screen-detected cancer risk
- Breast density is a better predictor of interval cancer risk
- This post is in direct response to the article entitled, “Deep Learning Predicts Interval and Screening-detected Cancer from Screening Mammograms: A Case-Case-Control Study in 6369 Women”, published in Radiology and presented at the Radiological Society of North America (RSNA)
Contents
- Why is Risk Important
- Current Risk Solutions
- Our Contribution Towards the Risk Solution
- Surprising Results
- What’s Next
The use of artificial intelligence (AI) and deep learning (DL) in the medical and healthcare field has been increasing at an astonishing rate. While the Health Insurance Portability and Accountability Act (HIPAA) is important for the protection of personal health information, it presented as the biggest barrier for gathering large data sets required for deep learning. Several strategies have been successfully implemented to gather lots of data for training medical AI systems without risking patient privacy. AI continues to have a significant impact on medical imaging and deep learning models are constantly being developed to look for anomalies such as bone fractures or possible cancer.
The introduction of breast cancer screening has helped to reduce cancer mortality rates in women as well as provide a consistent source of image data. Typically, women 40 years and older receive biannual or annual screening mammograms to check for any signs of cancer. Many have developed AI to detect and segment cancers within a mammogram yet, fewer have developed image based deep learning models to predict risk. In the work being described, we developed a model to predict an individual’s breast cancer risk; more specifically interval and screen-detected cancer risk.
Why is Risk Important?
Predicting breast cancer risk is analogous to forecasting which is an innately hard problem in all fields of science. By comparison, accurately quantifying a patient’s risk of cancer is more difficult than detecting the presence of cancer through some diagnostic test like a mammogram. Understanding breast cancer risk and the many factors associated with it is important for cancer prevention and monitoring strategies. The best way to reduce cancer mortality is to prevent cancer from developing in the first place and accurate assessments of risk are essential.
There are three clinically relevant outcomes when evaluating risk within a screening population. These outcomes include screen-detected (case), interval (case), or no (control) cancer risk. A screen-detected cancer is a cancer that is found as a result of a routine screening mammogram. In our study, we further defined it as a cancer that occurred within 12 months after a positive screening mammogram. An interval cancer is a cancer that is found between the normal screening interval (biannual or annual) and was defined in our study as an invasive cancer that occurred within 12 months after a negative screening mammogram. Interval cancers are known to grow more rapidly and be more aggressive in biology. They are often found through palpation or self examination.
Current Risk Solutions
The Gail, BRCAPRO, Tyrer-Cuzick, and Breast Cancer Surveillance Consortium (BCSC) risk models are examples of established models used clinically. They all use varying combinations of known breast cancer risk factors which can include age, body mass index (BMI), and/or breast density. Imaging is not directly used in clinical models and oftentimes only used to get better measurements of breast density. A few image based AI models have been published demonstrating comparable risk predictions performance. However, these AI models were constrained to a binary classification and did not include the three possible outcomes within a screening population.
Our Contribution Towards the Risk Solution
Our dataset consisted of 6,369 women recruited from the Mayo Clinic and the University of California at San Francisco. Four standard mammographic views which include the craniocaudal (CC) and mediolateral oblique (MLO) views for both the left and right breast were acquired on each woman. Mammographic images were received in their raw or “for-processing” format with pixel ranges between 0 and 214. Custom built software was used to filter the image into the “for-presentation” format that radiologists are used to reading. It is important to reiterate that the images used for our risk modeling were acquired at least 6 months prior to cancer diagnosis. Thus the images we use for modeling were negative and contained no visible cancers as determined by expert radiologists.
Model development took place at the University of Hawaii Cancer Center. A self imposed blind was implemented to account for possible overfitting. The data was split into a train and hold-out test set and the test set was never sent to the University of Hawaii. We further split the training set into a train, validate, and test set for our initial model development. Once we were confident in our modeling architecture and hyperparameters were optimized, the final model was trained on the entire training set. The final risk model was then sent to our collaborates to be evaluated on the hold-out test set. The process was very kaggle-esque.
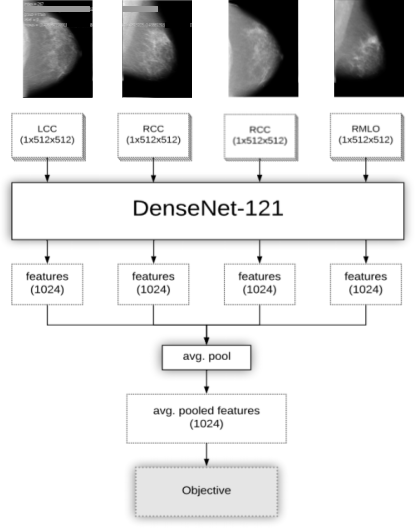
Figure 1: Four parallel networks for each mammographic view
The novelty of our deep learning model resulted from using four parallel networks to look at all imaging information simultaneously for prediction, seen in Figure 1. Other than density, imaging biomarkers of risk are underexplored and not well characterized. Although cancer may develop in only one side of the breast, we suspected signals of risk may be present throughout both the ipsilateral and contralateral breast. Each of the four networks was responsible for learning the information in one of the four mammographic views and so there was a LCC, RCC, LMLO, and RMLO network. Outputs from all networks were pooled and used to make final predictions of risk.
Surprising Results!
Using an objective versus else approach, we evaluated risk predictions using area under the receiver operating characteristic curve (AUC). Our model performed with an AUC of 0.66 when classifying controls verse everything else, 0.63 when classifying screen-detected cancer verse everything else, and 0.71 when classifying interval cancer verse everything else. See Figure 2. Risk modeling is a hard problem and these results are on par, if not better, than current clinical risk models.
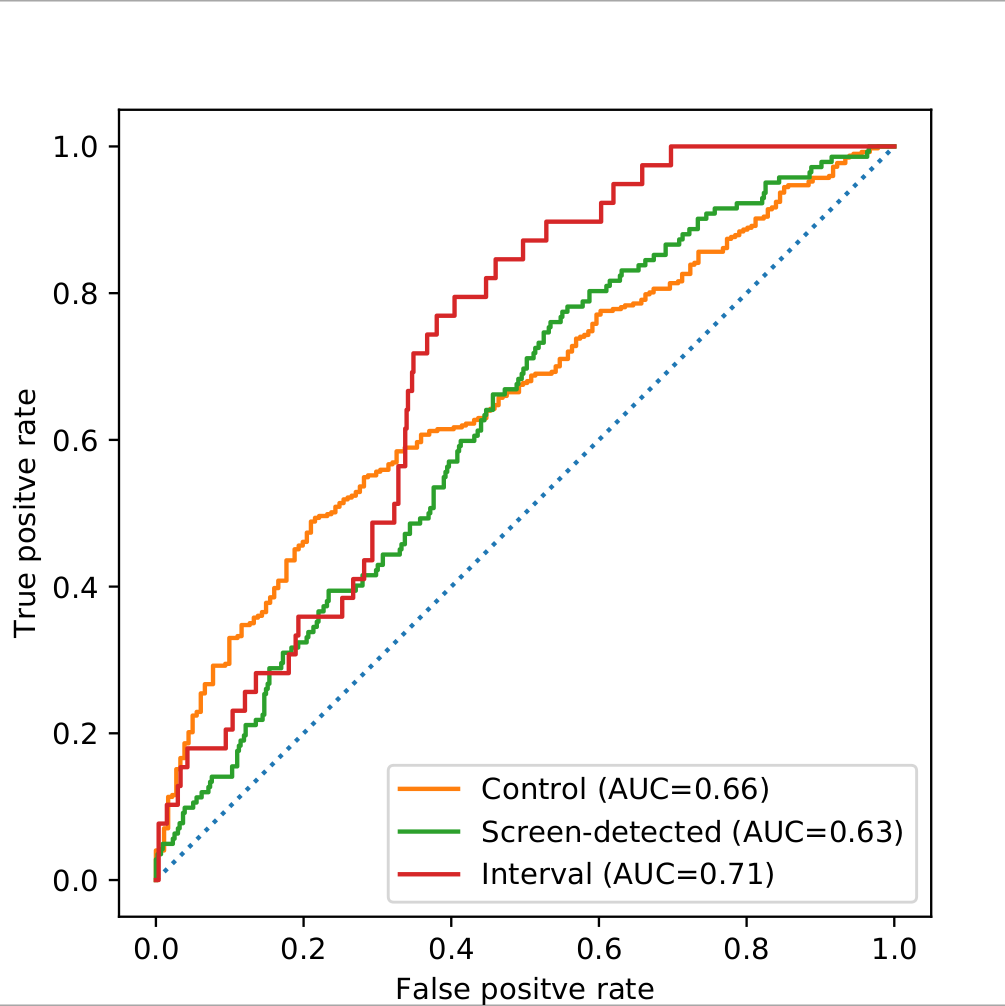
Figure 2: Final model performance on hold-out test set measured by area under the receiver operating characteristic curve (AUC)
To further interrogate possible imaging signals of risk, we compared our AI models performance against conditional logistic regression models built using common clinical risk factors. These risk factors included clinical and automated Breast Imaging Reporting and Data System (BI-RADS), BMI, and dense volume. Automated measurements were obtained using Volpara which is a clinically approved breast density software. In the screen-detected cancer case, c-statistics improved when combining risk factors with deep learning, shown in Figure 3. This improvement indicated that deep learning was able to pick up on imaging signals related to risk that are orthogonal or unique to clinical risk factors. Although we hypothesized it, it was still surprising! In the interval cancer case, deep learning models were not able to outperform models built on breast density alone. In other words, breast density remains the most powerful predictor of interval cancer risk. This was even more surprising!! Breast density is a risk factor because dense tissue can obscure images and mask possible lesions. Dense tissue may also be masking possible signals of risk and impairing our AI models performance.
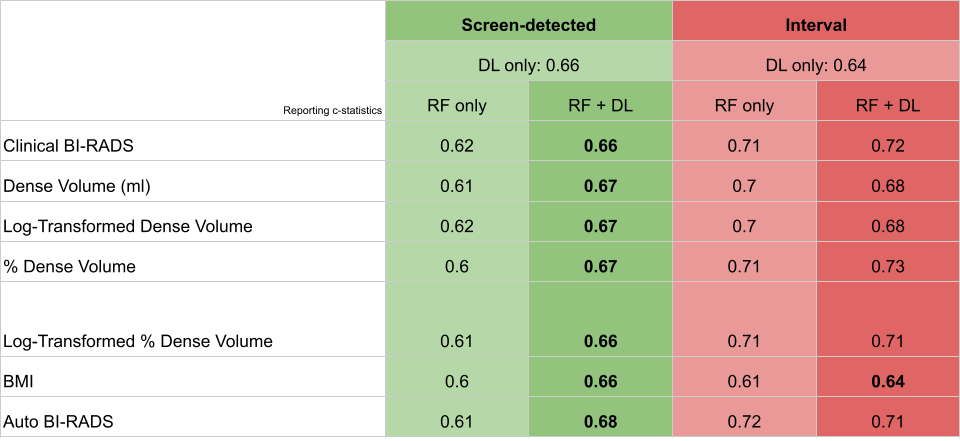
Figure 3: Deep learning performance compared against clinical risk factor models
What’s Next
Disentangling the density signal away from possible interval cancer risk signals is a possible future direction of this research. There are more advanced AI techniques, such as adversarial approaches, that may be helpful for understanding the interplay between breast density, imaging, and interval cancer risk. Hawaii has a higher incidence of late stage cancer when compared to the rest of the United States. The mechanism is not yet known but many hypothesize that the unique ethnic makeup of the population plays a role. Our success with deep learning in this study gives us confidence that predicting risk of late stage cancer is possible. We welcome comments, conversations, discussion, and collaboration on topics discussed here. Feel free to reach out and join the fight against cancer.
Continuing the Discussion
