Area Under the Curve and Beyond with Integrated Discrimination Improvement and Net Reclassification
Contents
TLDR
- AUC is a good starting metric when comparing the performance of two models but it does not always tell the whole story
- NRI looks at the new models ability to correctly reclassify event and nonevents and should be used alongside AUC
- IDI quantifies improvement of the slopes of the discrimination curves and plotting it can provide information AUC alone does not afford.
Area Under the Curve (AUC)
In machine learning and diagnostic medicine the area under the receiver operating characteristic (ROC) curve or AUC is a common metric used to evaluate the predictive performance of a model or diagnostic test. New models are often bench marked against established models using AUC. Comparing AUCs of new and old models to evaluate improvement is a good place to start however, many end their analysis here and believe that simply reporting a higher AUC is sufficient. AUC can be misleading as it gives equal weight to the full range of sensitivity and specificity values even though a limited range, or specific threshold, may be of practical interest. In this article, we show how to fully interrogate new and improved model performance, beyond simple AUC comparisons, as to provide a more comprehensive understanding of improvements within the context of a given problem. We also present a coded example, in Python, to demonstrate the concepts we present.
Example: Breast Cancer
Code for the following example and useful AUC, NRI, IDI functions can be found at github.
Breast cancer is the leading cause of cancer death in women world wide. Early detection has helped to lower the mortality rate and the earlier a malignancy is identified, the more likely a patient is to survive. As such, great effort has been allocated to developing predictive models to better identify cancer. In this example we use extracted imaging features to build models to predict malignancy probability. We use data from the Diagnostic Wisconsin Breast Cancer Database [1] housed at the UCI Machine Learning Repository.
As mentioned previously, AUC gives equal weight to all thresholds but this may not be practical in the context of breast cancer diagnosis. The Breast Imaging Reporting and Data System or (BI-RADS) [2]. provides a course of action for a given probability of malignancy. In short, if the probability of malignancy is greater than 2%, a biopsy is recommended. Biopsies are invasive procedures and they can be physically and mentally detrimental to patients. A new and improved breast model would ideally be better at identifying cancers (sensitivity increase) and reducing false positives (specificity increase), preferably below 2% to avoid invasive and unnecessary biopsies.
We set up the example with the following code snippets.
Example Set up
# Import Modules #
import os
import sys
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
import matplotlib.gridspec as gridspec
import seaborn as sns
from sklearn.ensemble import RandomForestClassifier
from sklearn.datasets import load_breast_cancer
from sklearn.model_selection import train_test_split
from sklearn import metrics
from sklearn.metrics import roc_auc_score
from sklearn.metrics import roc_auc_score
After we import the necessary modules, we load the breast cancer data set and split the data into a train and test set. Note, it is best practice to also create a validation set and we would normally do that but for the sake of this example we will stick with just a training and test set.
# Import data #
data = load_breast_cancer()
# Create DataFrame and Split Data
df = pd.DataFrame(data=data['data'],columns=data['feature_names'])
df['label']=data['target']
x_train, x_test, y_train, y_test = train_test_split(df.iloc[:,:-1], df.iloc[:,-1], test_size=0.40, random_state=123)
Also for the sake of this example, we will pretend that our reference/bench mark model was built on breast imaging features pertaining to texture, concave points, smoothness, fractal dimension, and compactness. We will also pretend that the new model uses additional imaging biomarkers/features which pertain to radius, perimeter, area, symmetry, and concavity. In total, the reference model is built using a total of 15 features while the new model is built using 30 features (15 features used by the original model and 15 new features). The following code snippet creates to two sets of features.
# create ref and new model feature sets #
ref_feat, new_feat = [],[]
for i in data['feature_names']:
if 'fractal' in i or 'smoothness' in i or 'texture' in i or 'concave' in i or 'compactness' in i:
ref_feat+=[i]
Modeling
We create a reference or “ref model” with 15 features and the “new model” with all 30 features.
# init models
ref_model = RandomForestClassifier()
new_model = RandomForestClassifier()
# fit models to train data
ref_model.fit(x_train[ref_feat], y_train)
new_model.fit(x_train[new_feat], y_train)
# make predictions on test data
test_ref_pred=ref_model.predict_proba(x_test[ref_feat])
test_new_pred=new_model.predict_proba(x_test[new_feat])
Issues with Comparing Models AUCs
We previously mentioned that comparing AUCs is a good starting point. We do that here to get an idea of how the new model performed with respect to our reference model. We use the following custom function to visualize ROC curves and confidence intervals (CI).
def bootstrap_results(y_truth, y_pred,num_bootstraps = 1000):
n_bootstraps = num_bootstraps
rng_seed = 42 # control reproducibility
y_pred=y_pred
y_true=y_truth
rng = np.random.RandomState(rng_seed)
tprs=[]
fprs=[]
aucs=[]
threshs=[]
base_thresh = np.linspace(0, 1, 101)
for i in range(n_bootstraps):
# bootstrap by sampling with replacement on the prediction indices
indices = rng.randint(0, len(y_pred), len(y_pred))
if len(np.unique(y_true[indices])) < 2:
# We need at least one positive and one negative sample for ROC AUC
continue
fpr, tpr, thresh = metrics.roc_curve(y_true[indices],y_pred[indices])
thresh=thresh[1:]
thresh=np.append(thresh,[0.0])
thresh=thresh[::-1]
fpr = np.interp(base_thresh, thresh, fpr[::-1])
tpr = np.interp(base_thresh, thresh, tpr[::-1])
tprs.append(tpr)
fprs.append(fpr)
threshs.append(thresh)
tprs = np.array(tprs)
mean_tprs = tprs.mean(axis=0)
fprs = np.array(fprs)
mean_fprs = fprs.mean(axis=0)
return base_thresh, mean_tprs, mean_fprs
def get_auc_ci(y_truth, y_pred,num_bootstraps = 1000):
n_bootstraps = num_bootstraps
rng_seed = 42 # control reproducibility
bootstrapped_scores = []
y_pred=y_pred
y_true=y_truth
rng = np.random.RandomState(rng_seed)
tprs=[]
aucs=[]
base_fpr = np.linspace(0, 1, 101)
for i in range(n_bootstraps):
# bootstrap by sampling with replacement on the prediction indices
indices = rng.randint(0, len(y_pred), len(y_pred))
if len(np.unique(y_true[indices])) < 2:
# We need at least one positive and one negative sample for ROC AUC
continue
score = roc_auc_score(y_true[indices], y_pred[indices])
bootstrapped_scores.append(score)
fpr, tpr, _ = metrics.roc_curve(y_true[indices],y_pred[indices])
roc_auc = metrics.auc(fpr, tpr)
aucs.append(roc_auc)
tpr = np.interp(base_fpr, fpr, tpr)
tpr[0] = 0.0
tprs.append(tpr)
tprs = np.array(tprs)
mean_tprs = tprs.mean(axis=0)
std = tprs.std(axis=0)
mean_auc = metrics.auc(base_fpr, mean_tprs)
std_auc = np.std(aucs)
tprs_upper = np.minimum(mean_tprs + std*2, 1)
tprs_lower = mean_tprs - std*2
return base_fpr, mean_tprs, tprs_lower, tprs_upper, mean_auc, std_auc
def plot_auc(truth, reference_model, new_model,n_bootstraps=1000, save=False):
y_truth = truth
ref_model = reference_model
new_model = new_model
ref_fpr, ref_tpr, ref_thresholds = metrics.roc_curve(y_truth, ref_model)
new_fpr, new_tpr, new_thresholds = metrics.roc_curve(y_truth, new_model)
ref_auc, new_auc = metrics.auc(ref_fpr, ref_tpr), metrics.auc(new_fpr, new_tpr)
print('ref auc =',ref_auc, '\newn auc = ', new_auc)
base_fpr_ref, mean_tprs_ref, tprs_lower_ref, tprs_upper_ref, mean_auc_ref, std_auc_ref=get_auc_ci(y_truth, ref_model,n_bootstraps)
base_fpr_new, mean_tprs_new, tprs_lower_new, tprs_upper_new, mean_auc_new, std_auc_new=get_auc_ci(y_truth, new_model,n_bootstraps)
plt.figure(figsize=(8, 8))
lw = 2
plt.plot(ref_fpr, ref_tpr, color='blue',
lw=lw, label='Reference raw ROC (AUC = %0.2f)' % ref_auc, linestyle='--')
plt.plot(base_fpr_ref, mean_tprs_ref, 'b', alpha = 0.8, label=r'Reference mean ROC (AUC=%0.2f, CI=%0.2f-%0.2f)' % (mean_auc_ref, (mean_auc_ref-2*std_auc_ref),(mean_auc_ref+2*std_auc_ref)),)
plt.fill_between(base_fpr_ref, tprs_lower_ref, tprs_upper_ref, color = 'b', alpha = 0.2)
plt.plot(new_fpr, new_tpr, color='darkorange',
lw=lw, label='New raw ROC (AUC = %0.2f)' % new_auc, linestyle='--')
plt.plot(base_fpr_new, mean_tprs_new, 'darkorange', alpha = 0.8, label=r'New mean ROC (AUC=%0.2f, CI=%0.2f-%0.2f)' % (mean_auc_new,(mean_auc_new-2*std_auc_new),(mean_auc_new+2*std_auc_new)),)
plt.fill_between(base_fpr_new, tprs_lower_new, tprs_upper_new, color = 'darkorange', alpha = 0.2)
plt.plot([0, 1], [0, 1], color='gray', lw=lw, linestyle='--')
plt.xlim([0.0, 1.0])
plt.ylim([0.0, 1.0])
plt.xlabel('1 - Specificity', fontsize=18)
plt.ylabel('Sensitivity', fontsize=18)
plt.legend(loc="lower right", fontsize=13)
plt.gca().set_aspect('equal', adjustable='box')
We run the following to generate AUC plots.
plot_auc(y_test.values,test_ref_pred[:,1],test_new_pred[:,1],n_bootstraps=100)
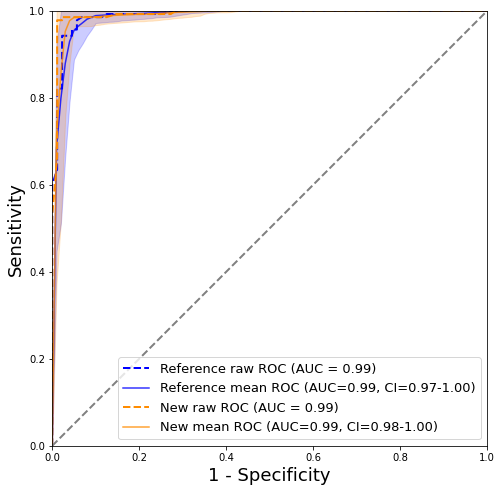
Figure 1: AUC curves with confidence intervals calculated using bootstrapping
Mean curves and the 95% confidence interval in Figure 1. were calculated via 100 rounds of bootstrapping, see code above. The reference (blue curve) and new model (orange curve) produce similar AUC at 0.99 and 0.99, respectively. The confidence interval is slightly narrower for the new model. Overall, it is difficult to draw any meaningful conclusion from the AUC curves alone and it would appear that the new model with additional features did little to nothing to improve malignancy predictions.
Beyond the Area Under the Curve
Our example in Figure 1. demonstrates how using only AUC can be limiting and suggest that analysis needs to go beyond simile AUC comparision. We present two additional metrics which are commonly used to asses the impact of new features or biomarkers on a model. These metrics include the net reclassification index (NRI) and the integrated discrimination improvement (IDI) [3]. These two metrics will help in understanding the actual differences in the two models that may not be obvious in Figure 1.
Net Reclassification Index (NRI)
The NRI is derived by summing the number of NRIevents and NRInonevents. In our example, events are synonymous to patients with cancer or maligancies and NRInonevents are synonymous to patients who do have benign findings. The NRIevents is the net proportion of patients with events reassigned to a higher risk category and the NRInonevents is the number of patients without events reassigned to a lower risk category [4]. We compute the NRI using the following Python functions.
def check_cat(prob,thresholds):
cat=0
for i,v in enumerate(thresholds):
if prob>v:
cat=i
return cat
def make_cat_matrix(ref, new, indices, thresholds):
num_cats=len(thresholds)
mat=np.zeros((num_cats,num_cats))
for i in indices:
row,col=check_cat(ref[i],thresholds),check_cat(new[i],thresholds)
mat[row,col]+=1
return mat
def nri(y_truth,y_ref, y_new,risk_thresholds):
event_index = np.where(y_truth==1)[0]
nonevent_index = np.where(y_truth==0)[0]
event_mat=make_cat_matrix(y_ref,y_new,event_index,risk_thresholds)
nonevent_mat=make_cat_matrix(y_ref,y_new,nonevent_index,risk_thresholds)
events_up, events_down = event_mat[0,1:].sum()+event_mat[1,2:].sum()+event_mat[2,3:].sum(),event_mat[1,:1].sum()+event_mat[2,:2].sum()+event_mat[3,:3].sum()
nonevents_up, nonevents_down = nonevent_mat[0,1:].sum()+nonevent_mat[1,2:].sum()+nonevent_mat[2,3:].sum(),nonevent_mat[1,:1].sum()+nonevent_mat[2,:2].sum()+nonevent_mat[3,:3].sum()
nri_events = (events_up/len(event_index))-(events_down/len(event_index))
nri_nonevents = (nonevents_down/len(nonevent_index))-(nonevents_up/len(nonevent_index))
return nri_events, nri_nonevents, nri_events + nri_nonevents
We input both the reference and new models predictions into the NRI function to calculate the events, nonevents, and total NRI.
print(nri(y_test.values,test_ref_pred[:,1],test_new_pred[:,1],[0.02,0.1,0.5,0.95]))
The output from the code above is:
0.1642857142857143, 0.125, 0.2892857142857143
It is tempting to state that “the new model reclassified 29% of the patients when compared to the reference model” however, this would not be an accurate statement nor a proper interpretation of NRI. Since the NRI is the sum of the proportion of events and not events it is possible to have an NRI of 2 or 200%. Stating that the new model reclassified 200% more individuals is not possible because it implies that there are suddenly twice as many patients.A more correct interpretation would be to state that the new model correctly classified 16% more cancer cases and 13% more benigns when compared to the reference, totaling a overall NRI of 29%.
The NRI show us that adding more features to the new model reclassified both malignant and non-malignant patients. This information could not be concluded from AUC alone and may have incorrectly led people to think the models were the same. In a clinical setting, the new model would have found more cancers which could potentially save more lives. Also, the new model would have correctly identified more benigns which would translate to less stress for an individual and potentially sparing them from having and invasive procedure like a biopsy.
Category free or cfNRI is a newer metric and tracks overall movement of event and nonevents, irrespective of class. For our breast cancer example, the American College of Radiology (ACR) has distinct BI-RADS classes and therefore we do not find the need to calculate the cfNRI. We provide coded functions to calculate cfNRIs below in the instance that it may suit you problem.
def track_movement(ref,new, indices):
up, down = 0,0
for i in indices:
ref_val, new_val = ref[i],new[i]
if ref_val<new_val:
up+=1
elif ref_val>new_val:
down+=1
return up, down
def category_free_nri(y_truth,y_ref, y_new):
event_index = np.where(y_truth==1)[0]
nonevent_index = np.where(y_truth==0)[0]
events_up, events_down = track_movement(y_ref, y_new,event_index)
nonevents_up, nonevents_down = track_movement(y_ref, y_new,nonevent_index)
nri_events = (events_up/len(event_index))-(events_down/len(event_index))
nri_nonevents = (nonevents_down/len(nonevent_index))-(nonevents_up/len(nonevent_index))
#print(events_up, events_down, len(event_index), nonevents_up, nonevents_down, len(nonevent_index), nri_events, nri_nonevents, nri_events+nri_nonevents)
return nri_events, nri_nonevents, nri_events + nri_nonevents
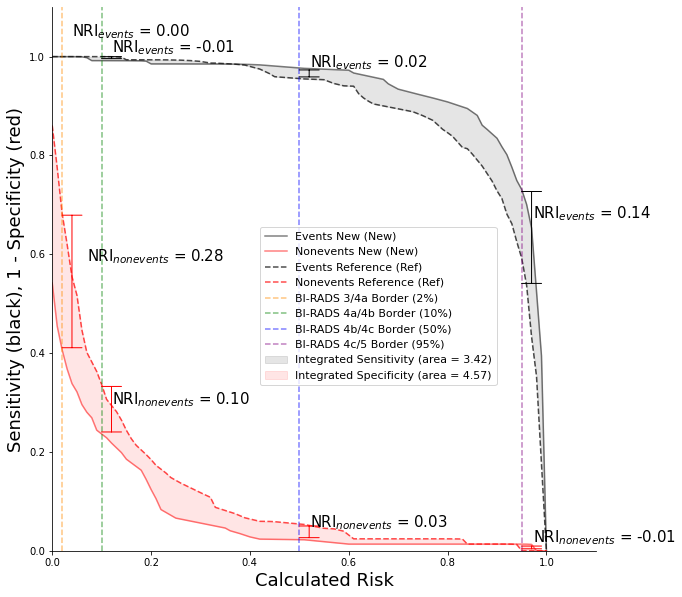
Integrate Discrimination Index (IDI)
The IDI is a measure in the change of the discrimination slopes and shows the impact of new biomarkers on a binary predictive model. The IDI is the sum of the integrated sensitivity (IS) and integrated specificity (IP) and like NRI, it separates out events and nonevents or in this case cancers and benigns. We use the following code to calculate and plot the IDI curves [5].
def area_between_curves(y1,y2):
diff = y1 - y2 # calculate difference
posPart = np.maximum(diff, 0)
negPart = -np.minimum(diff, 0)
posArea = np.trapz(posPart)
negArea = np.trapz(negPart)
return posArea,negArea,posArea-negArea
def plot_idi(y_truth, ref_model, new_model, save=False):
ref_fpr, ref_tpr, ref_thresholds = metrics.roc_curve(y_truth, ref_model)
new_fpr, new_tpr, new_thresholds = metrics.roc_curve(y_truth, new_model)
base, mean_tprs, mean_fprs=bootstrap_results( y_truth, new_model,100)
base2, mean_tprs2, mean_fprs2=bootstrap_results( y_truth, ref_model,100)
is_pos,is_neg, idi_event=area_between_curves(mean_tprs,mean_tprs2)
ip_pos,ip_neg, idi_nonevent=area_between_curves(mean_fprs2,mean_fprs)
print('IS positive', round(is_pos,2),'IS negative',round(is_neg,2),'IDI events',round(idi_event,2))
print('IP positive', round(ip_pos,2),'IP negative',round(ip_neg,2),'IDI nonevents',round(idi_nonevent,2))
print('IDI =',round(idi_event+idi_nonevent,2))
plt.figure(figsize=(10, 10))
ax=plt.axes()
lw = 2
plt.plot(base, mean_tprs, 'black', alpha = 0.5, label='Events New (New)' )
plt.plot(base, mean_fprs, 'red', alpha = 0.5, label='Nonevents New (New)')
plt.plot(base2, mean_tprs2, 'black', alpha = 0.7, linestyle='--',label='Events Reference (Ref)' )
plt.plot(base2, mean_fprs2, 'red', alpha = 0.7, linestyle='--', label='Nonevents Reference (Ref)')
plt.fill_between(base, mean_tprs,mean_tprs2, color='black',alpha = 0.1, label='Integrated Sensitivity (area = %0.2f)'%idi_event)
plt.fill_between(base, mean_fprs,mean_fprs2, color='red', alpha = 0.1, label='Integrated Specificity (area = %0.2f)'%idi_nonevent)
#''' #TODO: comment out if not for breast birads
### BIRADS Thresholds ###
plt.axvline(x=0.02,color='darkorange',linestyle='--',alpha=.5,label='BI-RADS 3/4a Border (2%)')
plt.axvline(x=0.10,color='green',linestyle='--',alpha=.5,label='BI-RADS 4a/4b Border (10%)')
plt.axvline(x=0.5,color='blue',linestyle='--',alpha=.5,label='BI-RADS 4b/4c Border (50%)')
plt.axvline(x=0.95,color='purple',linestyle='--',alpha=.5,label='BI-RADS 4c/5 Border (95%)')
def nri_annotation(plt, threshold):
x_pos = base[threshold]
x_offset=0.02
x_offset2=x_offset
text_y_offset=0.01
text_y_offset2=text_y_offset
if threshold==2:
text_y_offset=0.04
text_y_offset2=0.04
x_offset2=0.05
print(x_pos+x_offset, (np.mean([mean_tprs2[threshold], mean_tprs[threshold]])+text_y_offset),
x_pos, (np.mean([mean_tprs2[threshold], mean_tprs[threshold]])))
text_y_events=np.mean([mean_tprs2[threshold], mean_tprs[threshold]])+text_y_offset
text_y_nonevents=np.mean([mean_fprs[threshold], mean_fprs2[threshold]])+text_y_offset2
plt.annotate('', xy=(x_pos+0.02, mean_tprs2[threshold+1]), xycoords='data', xytext=(x_pos+0.02,
mean_tprs[threshold]), textcoords='data', arrowprops={'arrowstyle': '|-|'})
plt.annotate('NRI$_{events}$ = %0.2f'%(mean_tprs[threshold]-mean_tprs2[threshold]),
xy=(x_pos+x_offset, text_y_events), xycoords='data',
xytext=(x_pos+x_offset, text_y_events),
textcoords='offset points', fontsize=15)
plt.annotate('', xy=(x_pos+0.02, mean_fprs[threshold]), xycoords='data', xytext=(x_pos+0.02,
mean_fprs2[threshold]), textcoords='data', arrowprops=dict(arrowstyle= '|-|',color='r'))
plt.annotate('NRI$_{nonevents}$ = %0.2f'%(mean_fprs2[threshold]-mean_fprs[threshold]),
xy=(x_pos+x_offset2, text_y_nonevents), xycoords='data',
xytext=(x_pos+x_offset2, text_y_nonevents),
textcoords='offset points', fontsize=15)
print('Threshold =',round(x_pos,2),'NRI events =',round(mean_tprs[threshold]-mean_tprs2[threshold],4),
'NRI nonevents =',round(mean_fprs2[threshold]-mean_fprs[threshold],4),'Total =',
round((mean_tprs[threshold]-mean_tprs2[threshold])+(mean_fprs2[threshold]-mean_fprs[threshold]),4))
nri_annotation(plt,2)
nri_annotation(plt,10)
nri_annotation(plt,50)
nri_annotation(plt,95)
#'''
plt.xlim([0.0, 1.10])
plt.ylim([0.0, 1.10])
ax.spines['right'].set_visible(False)
ax.spines['top'].set_visible(False)
plt.xlabel('Calculated Risk', fontsize=18)
plt.ylabel('Sensitivity (black), 1 - Specificity (red)', fontsize=18)
plt.legend(loc="upper right", fontsize=11)
plt.legend(loc=0, fontsize=11, bbox_to_anchor=(0,0,1.2,.9))
plt.gca().set_aspect('equal', adjustable='box')
#if save:
# plt.savefig('idi_curve.png',dpi=300, bbox_inches='tight')
look=95
plt.show()
We run the following command to generate the graph in Figure 2.
plot_idi(y_test.values,test_ref_pred[:,1],test_new_pred[:,1])
Output:
IS positive 3.58 IS negative 0.16 IDI events 3.42
IP positive 4.61 IP negative 0.04 IDI nonevents 4.57
IDI = 7.98
0.04 1.04 0.02 1.0
Threshold = 0.02 NRI events = 0.0 NRI nonevents = 0.2762 Total = 0.2762
Threshold = 0.1 NRI events = -0.008 NRI nonevents = 0.1002 Total = 0.0922
Threshold = 0.5 NRI events = 0.0215 NRI nonevents = 0.0317 Total = 0.0532
Threshold = 0.95 NRI events = 0.1389 NRI nonevents = -0.0136 Total = 0.1252

Figure 2: IDI curve with calculated NRI at each class or BI-RADS border
The dashed and solid curves represent the reference and new model respectively. In the ideal case, the solid black line would be moved to the upper right indicating the most improvement to sensitivity and the red line would be lowered to the bottom right indicating the most improved specificity. The area between the black and red curves equate to the IS and IP and the total sum of the black and red area equates to the IDI. IDI provides more information that the AUC curves especially with respects to the orange dashed vertical line or the BI-RADS 3/4a border. At this border, the IP or red area is large which indicates that the new model was able to better predict benigns. This border is particularly interesting because it is recommended that all patients with BI-RADS 4 or greater receive biopsies [6]. The large area at the 3/4a border shows that adding new features to the new model increase specificity and potentially prevented 28% more people (NRInonevents=0.28) from being unnecessarily biopsied.
Final Thoughts
AUC is a good metric but does not provide all the information needed for a comprehensive analysis of the impact of new biomarkers and models. The NRI and IDI should be used to complement AUC findings and interpretations. As a data scientist and cancer researcher who uses Python, it was difficult to find functions and code snippets that computed and plotted NRI and IDI as presented here. This was my motivation for posting this and I hope that my code can help other in their research, discoveries, and pursuit of better health care solutions.
References
[1] Dua, D. and Graff, C, (2019), Irvine, CA: University of California, School of Information and Computer Science, UCI Machine Learning Repository [http://archive.ics.uci.edu/ml].
[2] Understanding mammogram reports: Mammogram results. (n.d.). Retrieved May 06, 2021, from https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/mammograms/understanding-your-mammogram-report.html
[3] Pencina, M. J., D’Agostino Sr, R. B., D’Agostino Jr, R. B., & Vasan, R. S., Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond, (2008), Statistics in medicine, 27(2), 157-172.
[4] Pencina, M. J., D’Agostino Sr, R. B., & Steyerberg, E. W., Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers, (2011), Statistics in medicine, 30(1), 11-21.
[5] Pickering, J. W., & Endre, Z. H., New metrics for assessing diagnostic potential of candidate biomarkers, (2012), Clinical Journal of the American Society of Nephrology, 7(8), 1355-1364.
[6] Leong, L., Malkov, S., Drukker, K., Niell, B., Sadowski, P., Wolfgruber, T., … & Shepherd, J., Dual-energy three compartment breast imaging (3CB) for novel compositional biomarkers to improve detection of malignant lesions, (2021).
